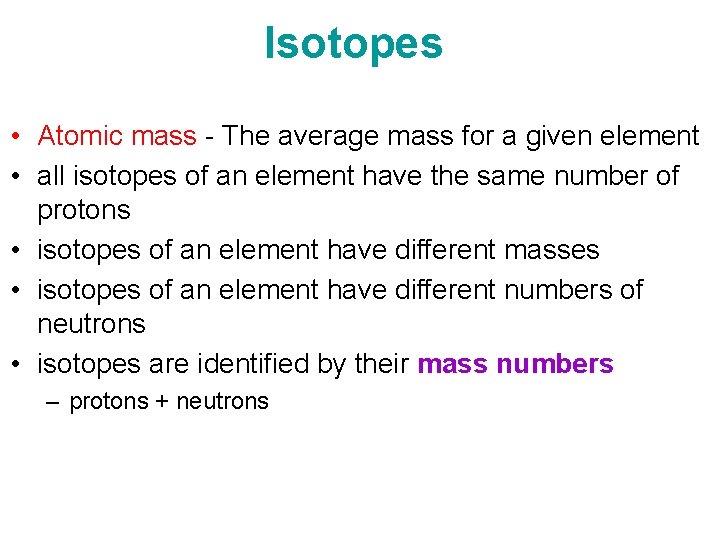
- Isotopes Of An Element Have What In Common
- Isotopes Of An Element Have The Same Number Of
- Isotopes Of An Element Have Quizlet
- Isotopes Of An Element Have The Same Number Of Protons
- Isotopes Of An Element Have Different Numbers Of Quizlet
- Isotopes Of An Element Have The Same Number
Atoms of the same element must have the same number of protons, but they can have different numbers of neutrons. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes of a given element have the same number of, but different numbers of in their nucleus. Neutrons, electrons b. Electrons, protons c. Protons, electrons d. Protons, neutrons 7. All of the following statements are true EXCEPT a. For any neutral element, the number of protons and electrons are equal. Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons. The number of protons in a nucleus determines the element’s atomic number on the Periodic Table. For example, carbon has six protons and is atomic number 6. Windows 10 and mac.
Learning Objective

- Discuss the properties of isotopes and their use in radiometric dating
Key Points
- Isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons.
- Despite having different numbers of neutrons, isotopes of the same element have very similar physical properties.
- Some isotopes are unstable and will undergo radioactive decay to become other elements.
- The predictable half-life of different decaying isotopes allows scientists to date material based on its isotopic composition, such as with Carbon-14 dating.
Terms
- isotopeAny of two or more forms of an element where the atoms have the same number of protons, but a different number of neutrons within their nuclei.
- half-lifeThe time it takes for half of the original concentration of an isotope to decay back to its more stable form.
- radioactive isotopesan atom with an unstable nucleus, characterized by excess energy available that undergoes radioactive decay and creates most commonly gamma rays, alpha or beta particles.
- radiocarbon datingDetermining the age of an object by comparing the ratio of the 14C concentration found in it to the amount of 14C in the atmosphere.
What is an Isotope?
Isotopes are various forms of an element that have the same number of protons but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have multiple naturally-occurring isotopes. Isotopes are defined first by their element and then by the sum of the protons and neutrons present.
- Carbon-12 (or 12C) contains six protons, six neutrons, and six electrons; therefore, it has a mass number of 12 amu (six protons and six neutrons).
- Carbon-14 (or 14C) contains six protons, eight neutrons, and six electrons; its atomic mass is 14 amu (six protons and eight neutrons).
While the mass of individual isotopes is different, their physical and chemical properties remain mostly unchanged.
Isotopes do differ in their stability. Carbon-12 (12C) is the most abundant of the carbon isotopes, accounting for 98.89% of carbon on Earth. Carbon-14 (14C) is unstable and only occurs in trace amounts. Unstable isotopes most commonly emit alpha particles (He2+) and electrons. Neutrons, protons, and positrons can also be emitted and electrons can be captured to attain a more stable atomic configuration (lower level of potential energy) through a process called radioactive decay. The new atoms created may be in a high energy state and emit gamma rays which lowers the energy but alone does not change the atom into another isotope. These atoms are called radioactive isotopes or radioisotopes.
Radiocarbon Dating
Isotopes Of An Element Have What In Common
Carbon is normally present in the atmosphere in the form of gaseous compounds like carbon dioxide and methane. Carbon-14 (14C) is a naturally-occurring radioisotope that is created from atmospheric 14N (nitrogen) by the addition of a neutron and the loss of a proton, which is caused by cosmic rays. This is a continuous process so more 14C is always being created in the atmosphere. Once produced, the 14C often combines with the oxygen in the atmosphere to form carbon dioxide. Carbon dioxide produced in this way diffuses in the atmosphere, is dissolved in the ocean, and is incorporated by plants via photosynthesis. Animals eat the plants and, ultimately, the radiocarbon is distributed throughout the biosphere.
In living organisms, the relative amount of 14C in their body is approximately equal to the concentration of 14C in the atmosphere. When an organism dies, it is no longer ingesting 14C, so the ratio between 14C and 12C will decline as 14C gradually decays back to 14N. This slow process, which is called beta decay, releases energy through the emission of electrons from the nucleus or positrons.
After approximately 5,730 years, half of the starting concentration of 14C will have been converted back to 14N. This is referred to as its half-life, or the time it takes for half of the original concentration of an isotope to decay back to its more stable form. Because the half-life of 14C is long, it is used to date formerly-living objects such as old bones or wood. Comparing the ratio of the 14C concentration found in an object to the amount of 14C in the atmosphere, the amount of the isotope that has not yet decayed can be determined. On the basis of this amount, the age of the material can be accurately calculated, as long as the material is believed to be less than 50,000 years old. This technique is called radiocarbon dating, or carbon dating for short.
Other elements have isotopes with different half lives. For example, 40K (potassium-40) has a half-life of 1.25 billion years, and 235U (uranium-235) has a half-life of about 700 million years. Scientists often use these other radioactive elements to date objects that are older than 50,000 years (the limit of carbon dating). Through the use of radiometric dating, scientists can study the age of fossils or other remains of extinct organisms.
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Carbon-12
Wikipedia
CC BY-SA 4.0.

http://en.wiktionary.org/wiki/isotope
Wiktionary
CC BY-SA 3.0.
Isotopes Of An Element Have The Same Number Of
http://en.wikipedia.org/wiki/Radiocarbon_dating
Wikipedia
CC BY-SA 3.0.
http://cnx.org/content/m44390/latest/?collection=col11448/latest
OpenStax CNX
CC BY 3.0.
Isotopes Of An Element Have Quizlet
http://cnx.org/content/m44390/latest/Figure_02_01_03.jpg
OpenStax CNX
CC BY 3.0.
A family of people often consists of related but not identical individuals. Elements have families as well, known as isotopes. Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons.
Wear os download music. The number of protons in a nucleus determines the element’s atomic number on the Periodic Table. For example, carbon has six protons and is atomic number 6. Carbon occurs naturally in three isotopes: carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes.
The addition of even one neutron can dramatically change an isotope’s properties. Carbon-12 is stable, meaning it never undergoes radioactive decay. Carbon-14 is unstable and undergoes radioactive decay with a half-life of about 5,730 years (meaning that half of the material will be gone after 5,730 years). This decay means the amount of carbon-14 in an object serves as a clock, showing the object’s age in a process called “carbon dating.”
Isotopes Of An Element Have The Same Number Of Protons
Isotopes have unique properties, and these properties make them useful in diagnostics and treatment applications. They are important in nuclear medicine, oil and gas exploration, basic research, and national security.
DOE Office of Science & Isotopes
Isotopes Of An Element Have Different Numbers Of Quizlet
Top 5 torrents. Isotopes are needed for research, commerce, medical diagnostics and treatment, and national security. However, isotopes are not always available in sufficient quantities or at reasonable prices. The DOE Isotope Program addresses this need. The program produces and distributes radioactive and stable isotopes that are in short supply, including byproducts, surplus materials, and related isotope services. The program also maintains the infrastructure required to produce and supply priority isotope products and related services. Finally, it conducts research and development on new and improved isotope production and processing techniques.
Isotope Facts
- All elements have isotopes.
- There are two main types of isotopes: stable and unstable (radioactive).
- There are 254 known stable isotopes.
- All artificial (lab-made) isotopes are unstable and therefore radioactive; scientists call them radioisotopes.
- Some elements can only exist in an unstable form (for example, uranium).
- Hydrogen is the only element whose isotopes have unique names: deuterium for hydrogen with one neutron and tritium for hydrogen with two neutrons.
Resources and Related Terms
- National Isotope Development Center (Isotope Basics)
Isotopes Of An Element Have The Same Number
Scientific terms can be confusing. DOE Explains offers straightforward explanations of key words and concepts in fundamental science. It also describes how these concepts apply to the work that the Department of Energy’s Office of Science conducts as it helps the United States excel in research across the scientific spectrum.
